List of obtained certificates

The Design, Development, Manufacture, Distribution, Installation, and Servicing of In-Vitro Diagnostic medical devices and supporting software used in detecting food IgG antibodies, including home use for sample collection kits, self-tests and near patient/point of care in-vitro diagnostic test kits.
ISO 13485:2016

-
The Design, Development, Manufacture, Distribution, Installation, and Servicing of In-Vitro Diagnostic medical devices and supporting software used in detecting food IgG antibodies, including home use for sample collection kits, self-tests and near patient/point of care in-vitro diagnostic test kits. Provision of Laboratory services for health and well-being diagnostic and screening tests.
ISO 13485:2016

-

The design, development and manufacture of in vitro diagnostic test kits, reagents and assessories.
ISO 13485:2016
 |
 |
-

The design, development and manufacture of in vitro diagnostic test kits, reagents and accessories.
ISO 13485:2016
 |
 |
-

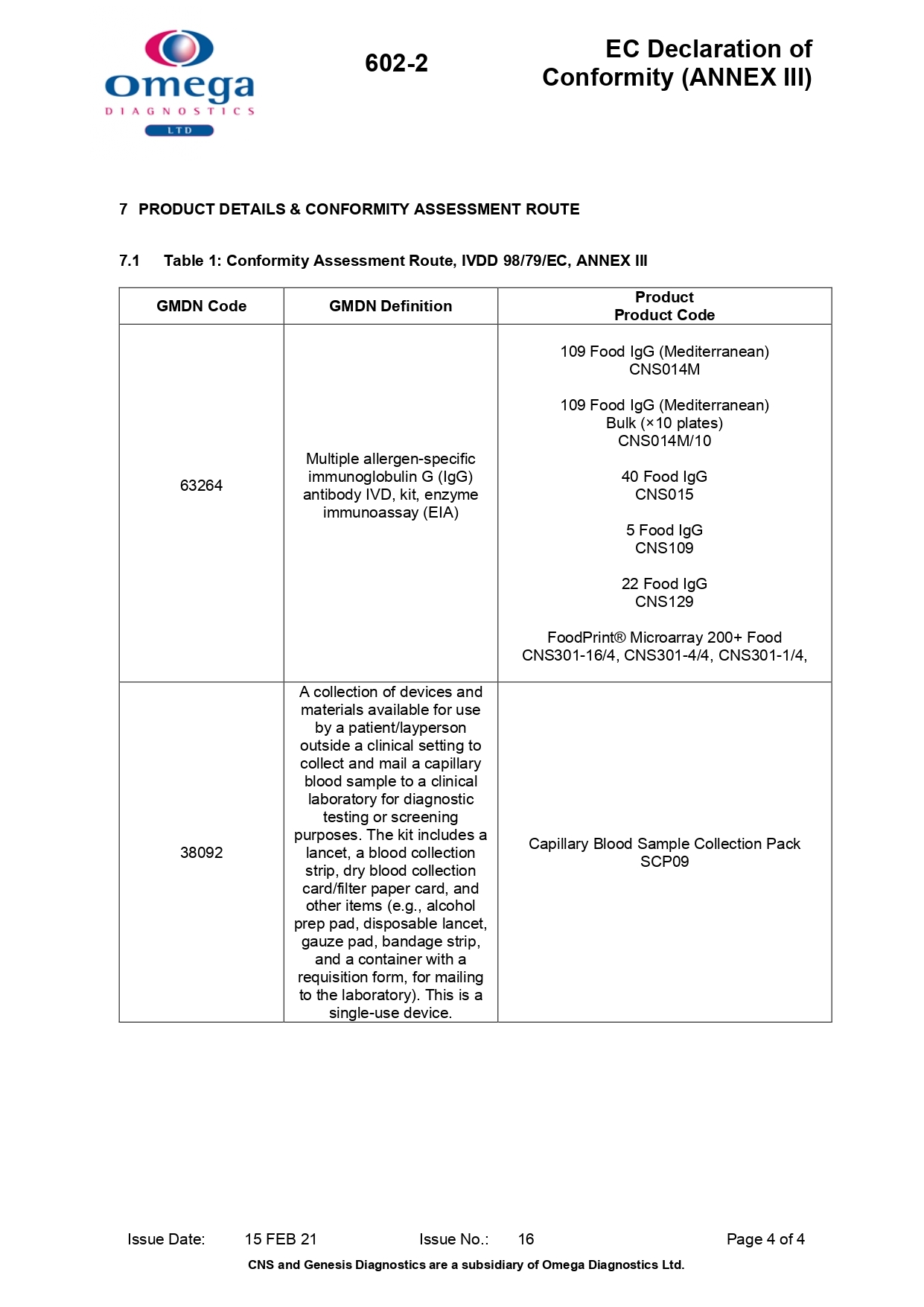
EC Declaration of Conformity (ANNEX III)
EN ISO 9001:2015 / EN ISO 13485:2016 / BE EN ISO 14971:2012
BS EN 13641:2002 / BS EN ISO 18113:2011 / BS EN ISO 23640:2015
 |
 |
 |
 |
-


